2021 Industry Innovators
Simple, Safe, Convenient & Effective Knee Extension Rehabilitation
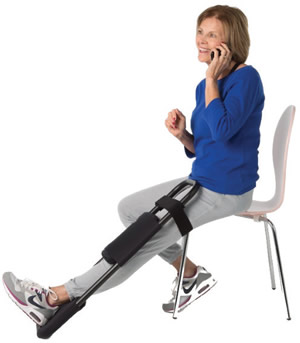 Kneewell, LLC is a durable
medical device design, development,
and manufacturing
company headquartered in Minnetonka,
Minnesota. It was established to
provide a simple, safe, convenient and
effective method for knee extension
rehabilitation. The flagship product,
Kneewell® is a patented rehab system
engineered to augment total knee
arthroplasty post-operative care. Our
extensive clinical results have demonstrated
that use of the Kneewell® significantly
accelerates return to function
following knee surgery.
Kneewell, LLC is a durable
medical device design, development,
and manufacturing
company headquartered in Minnetonka,
Minnesota. It was established to
provide a simple, safe, convenient and
effective method for knee extension
rehabilitation. The flagship product,
Kneewell® is a patented rehab system
engineered to augment total knee
arthroplasty post-operative care. Our
extensive clinical results have demonstrated
that use of the Kneewell® significantly
accelerates return to function
following knee surgery.
The Problem
Knee arthroscopic surgery often leaves
patients with knee contractures that are
very stubborn to resolve. Many hours of
intensive physical therapy often result in
only partial results, leaving the patient
unable to fully extend their leg. Without
complete extension, activities like golf,
tennis, or even walking can be painful.
The remaining degrees of extension
prove resilient to conventional therapies
because the soft tissue requires more
prolonged stretch than is possible with
PT treatment alone.
The Treatment
Using the Kneewell® allows the patient
to self-administer extension therapy
at home or work, as frequently as
required. Regularly stretching the soft
tissues has proven to be effective in
retaining therapeutic gains resulting in
permanent recovery. Because they have
access to therapy between sessions,
the Kneewell® is an excellent addition
to physical therapy to retain and
enhance progress achieved in the clinic.
The device is easy to use, lightweight
and durable, making it easy to adopt
for at home use. Using the Kneewell®
makes therapy part of patients everyday
routine. The patient controls the
intensity of the load, minimizing pain
and encouraging regular use.
Results
Providers find that patients resolve
their contractures more quickly and
with reduced use of pain medication.
The device helps patients reduce pain,
recover more quickly and gain those
last several degrees of knee extension.
Patients augmenting treatment with the
Kneewell® between therapy sessions
return to fun, function and their normal
daily routines.
Reimbursement
The Kneewell® has been registered
with the FDA as a Class 1 device and
Medicare has issued the reimbursement
code E1811. The device may be billed to
Medicare as a static progressive stretch
knee device used for extension. This
code is a capped rental code and therefore
the device can be used as long as
is necessary, though most patients have
complete resolution of their contracture
within two to three months. Having this
reimbursement available makes the
Kneewell® easily attainable for everyone,
provides all patients the opportunity
to accelerate their therapy and
return to normal activities more quickly
after knee surgery.
Our Company
Our mission at Kneewell is “to develop
and distribute quality rehabilitation
products to the orthopedic industry
and provide the customers with educational
support about their rehabilitation
as well as a quality product”. We are
committed to ensuring that customers
are satisfied with the product as well as
the results that the Kneewell® can help
them achieve. Our company is committed
to producing a quality product that
enhances our customers lives.
Kneewell® enables patients with
disabilities or advanced age to use the
device at home with little or no aid and
has proven to be remarkably effective
on medium and high-risk patients. The
product is endorsed by surgeons, therapists,
and patients. Use of the Kneewell
can restore full extension to Knee
arthroscopic surgery patients. This provides
patients freedom of movement
and a return to their active lifestyle. The
Kneewell®’s lightweight design means
patients can easily and safely perform
knee therapy at home, and bring it with
them wherever they go.
KNEEWELL LLC
Office: (952) 546-5334
Toll-Free: (800) 736-8367
Fax: (952) 546-2657
[email protected]
www.kneewell.com
This article originally appeared in the Jul/Aug 2021 issue of HME Business.